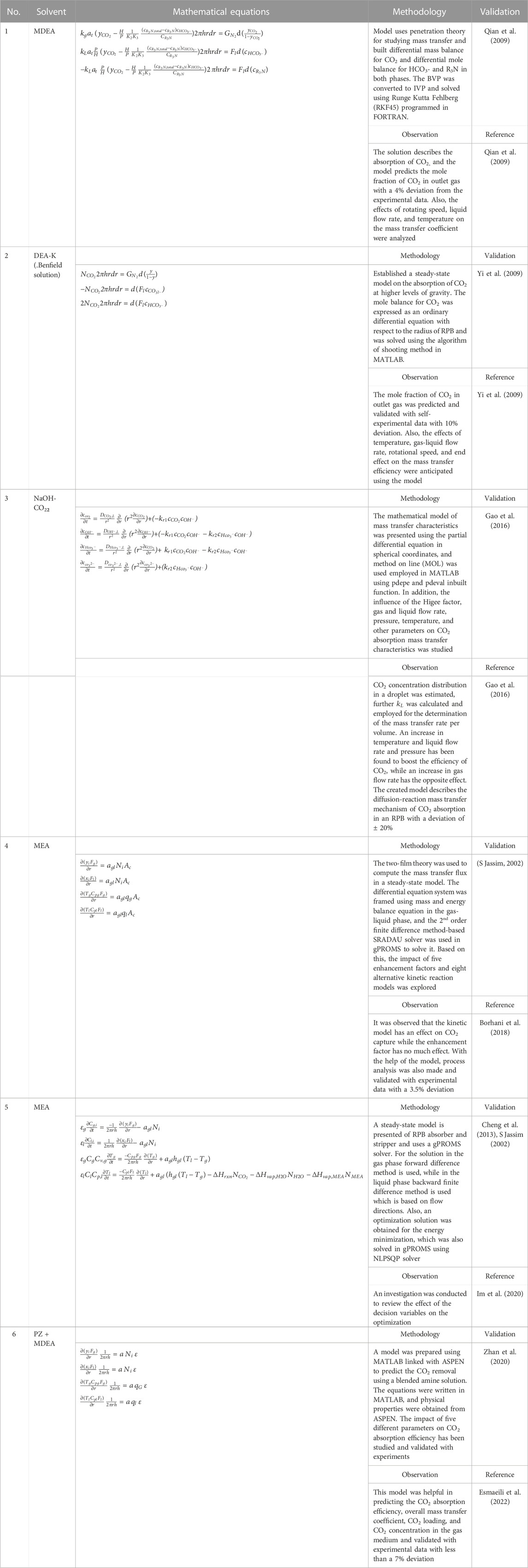
A review of process intensified CO2 capture in RPB for sustainability and contribution to industrial net zero
- 1Department of Mathematics, Pandit Deendayal Energy University, Gandhinagar, Gujarat, India
- 2Department of Chemical Engineering, (CO2 Research Group) Pandit Deendayal Energy University, Gandhinagar, Gujarat, India
Carbon dioxide (CO2), a significant greenhouse gas released from power plants and industries, substantially impacts climate change; minimizing it and achieving carbon net zero is essential globally. In the direction of reducing CO2 emissions into the atmosphere, post-combustion carbon capture from large point CO2 emitters by chemical absorption involving the absorption of this gas in a capturing fluid is a commonly used and efficacious mechanism. Researchers have worked on the process using conventional columns. However, process intensification technology is required because of the high capital cost, the absorption column height, and the traditional columns’ low energy efficiency. Rotating packed bed (RPB) process intensification equipment has been identified as a suitable technology for enhanced carbon capture using an absorbing fluid. This article reviews and discusses recent model developments in the post-combustion CO2 capture process intensification using rotating packed beds. In the literature, various researchers have developed steady-state mathematical models regarding mass balance and energy balance equations in gas and liquid phases using ordinary or partial differential equations. Due to the circular shape, the equations are considered in a radial direction and have been solved using a numerical approach and simulated using different software platforms, viz. MATLAB, FORTRAN, and gPROMS. A comparison of various correlations has been presented. The models predict the mole fraction of absorbed CO2 and correspond well with the experimental results. Along with these models, an experimental data review on rotating packed bed is also included in this work.
1 Introduction
The most challenging task currently is to control the increased levels of greenhouse gases (GHGs) in the atmosphere, which leads to global warming. Among the various GHGs, carbon dioxide (CO2) significantly affects global warming. GHGs are being released into the atmosphere through multiple activities such as fuel combustion, manufacturing, construction, industrial process, transportation, and agriculture. CO2 emissions from factories and power plants are the most significant sources of atmospheric pollution. Following the International Panel on Climate Change (IPCC) report, the CO2 level will have increased to 570 ppm in the atmosphere by 2,100. This CO2 level will cause an increase in global temperature of approximately 1.9°C. (IPCC, 2022). There is a need to reduce these emissions of GHGs, and hence carbon capture, storage, and utilization (CCSU) is a solution. Though various technology options are available, such as pre-combustion, oxyfuel combustion, and post-combustion carbon capture (PCC), post-combustion is the widely preferred method as it can be used in the pre-existing plant compared to the other two methods (Marx-Schubach and Schmitz, 2019). The technologies available for the post-combustion process include membrane separation, solvent absorption, cryogenic fractionation, and adsorption using solid sorbent (Afkhamipour and Mofarahi, 2013; Dey et al., 2018). Chemical absorption is a widely observed post-combustion method used for capturing carbon compared to other techniques due to its high absorption rate (Joel et al., 2014).
The desirable properties of a suitable solvent are high thermodynamic capacity, high absorption rate, low energy for regeneration, less degradation, lower volatility, and lower corrosivity (Dey et al., 2020). Due to its desirable properties, the solvent used in industry is an amine-based solvent such as primary, secondary, tertiary, and sterically hindered amines (H. Li et al., 2013).
For years, researchers have studied post-combustion CO2 capture through chemical absorption using different solvents in the conventional column. These include aqueous solutions of monoethanolamine (MEA), diethanolamine (DEA), methyl diethanolamine (MDEA), 2-amino-2-methyl-1-propanol (AMP), and blended amine AMP and piperazine. The most widely used alkanolamines in the literature include MEA, DEA, and MDEA (Dash et al., 2012). Currently, many experiments have been conducted using blended amine AMP and piperazine or its derivatives (Dash et al., 2022; M; Wang et al., 2011; Wu et al., 2020). Mathematical models and operational parameters of the conventional absorber have been studied, modeled, and simulated in various research papers (Pandya, 1983; Akanksha et al., 2007; Kvamsdal et al., 2009; Harun et al., 2012; Koronaki et al., 2015; Jin et al., 2019; Shahid et al., 2021). However, the large size of the conventional column has been cited as a hindrance to CO2 capture. The problems are that it demands significant capital and operating costs, uses a lot of energy, and requires intercooling with heat integration (Chamchan et al., 2017). Thus, more efficient gas-liquid reactors employing new principles and technology for carbon dioxide capture are needed.
Process intensification technology is used to reduce capital costs and significantly improve process dynamics. The equipment can be made smaller using centrifugal forces, electrical fields, and microwaves or by downsizing to the meso- or microscale (Reay, 2008; Cortes Garcia et al., 2017). One of the most cutting-edge intensification technologies invented by Mallinson and Ramshaw (Mallinson and Ramshaw, 1981) is currently the rotating packed bed (RPB). Podbielniak, (1935) introduced the concept of reducing the height of the distillation column and designed a conceptual contactor for distillation. Mallinson and Ramshaw introduced the idea of rotating packed bed columns similar to that proposed by Pilo and Dahlbeck for distillation and absorption (Rao, 2022). The RPB is used in place of a packed bed absorber to capture CO2 (M. Wang et al., 2015).
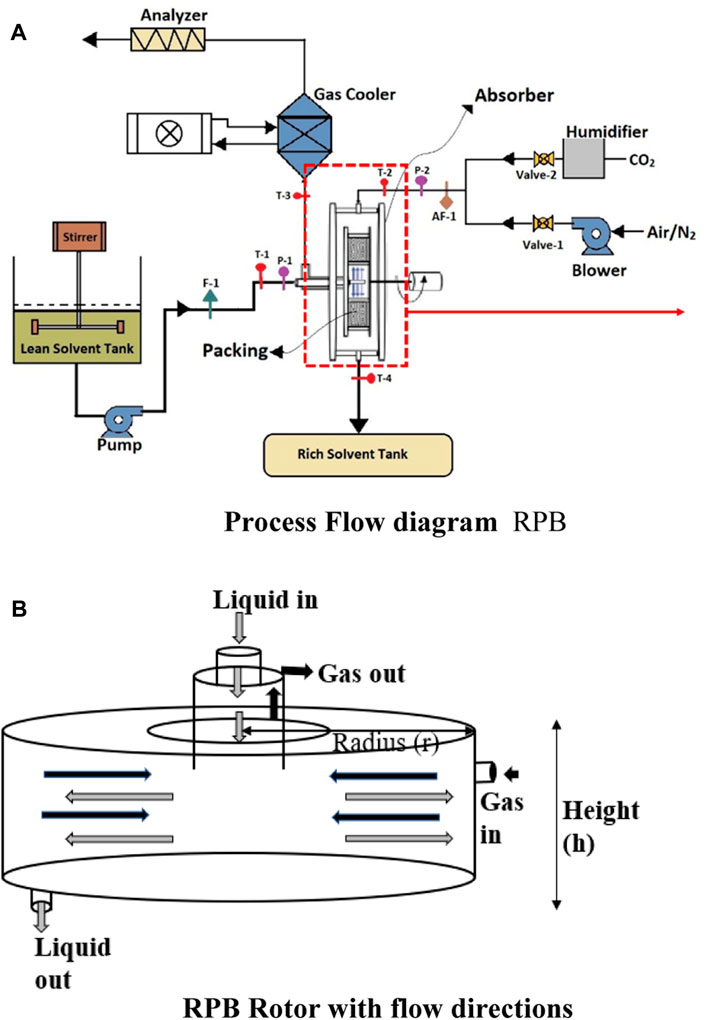
An RPB has been designed and installed at our laboratory to make a complete pilot plant for CO2 capture. The process flow diagram of the RPB shown in Figure 1A, has the appearance of a doughnut, which spins to produce acceleration caused by centrifugal force. This force is far better than the acceleration caused by gravity applicable in conventional absorption columns for the promotion of micromixing and enhanced mass transfer. The spinning packed bed consists of a rotor with packing that has a considerable surface area and is shaped like a doughnut. A schematic diagram of the rotor is presented in Figure 1B. The solvent is first supplied to the rotor’s center. It is forced by the rotor force to flow in the circular direction via the space between the packing. Under the influence of the pressure gradient, the gas flow occurs concurrently in the direction opposite to the flow of the solvent. When the shaft is connected to a motor, the rotor may be rotated at high rates, creating significant gravitational acceleration. Using centrifugal force, a liquid distributor may transport the liquid to the packing’s inner edge (Ayash and Mahmood, 2022). This enhances the efficiency of mass transfer and is also an essential component of process intensification.
An RPB works on the principle of centrifugal acceleration (
However, RPB equipment is associated with more significant maintenance and repair expenses. Furthermore, the design of an RPB is more complex; design methodologies and relevant models are not as well defined as conventional packed columns. Despite this, an RPB is favored for CO2 capture over a packed bed column as the HiGee technology has more advantages. Because of an RPB’s high-efficiency mass transfer performance and reduction in size in comparison to the conventional column, it has wide industrial applications. These applications are selective removal of H2S, desulphurization, dedusting, and deaeration of water, as well as an application in the pharmaceutical industry where higher purity of a product is required (Neumann et al., 2018).
Because of these benefits, it is good to have high-quality research on CO2 capture with RPB absorbers through different experiments and modeling approaches to meet desirable environmental requirements. CO2 capturing with RPB absorbers is comparatively unexplored and efficient. Thus, it has significant scope for research using different experiments and modeling approaches to meet desirable environmental requirements. Detailed and systematic modeling of RPBs is essential for large-scale CO2 capture applications because experimental work is a long process and is limited. One can be robust in operation through modeling. Here, RPB models proposed by researchers during the last few years are reviewed and analyzed to predict CO2 absorption efficiency using different characteristics and modeling approaches.
In this work, the first section reviews the different modeling approaches used in CO2 capture utilizing RPB. The second contains sub-sections summarizing the study of hydrodynamic characteristics, the study of correlations of mass transfer characteristics of RPB, CO2 capture modeling and process simulation using RPB, experimental review, and techno-economic analysis. The third section presents a conclusion and proposes fields for future work.
2 Modeling approaches
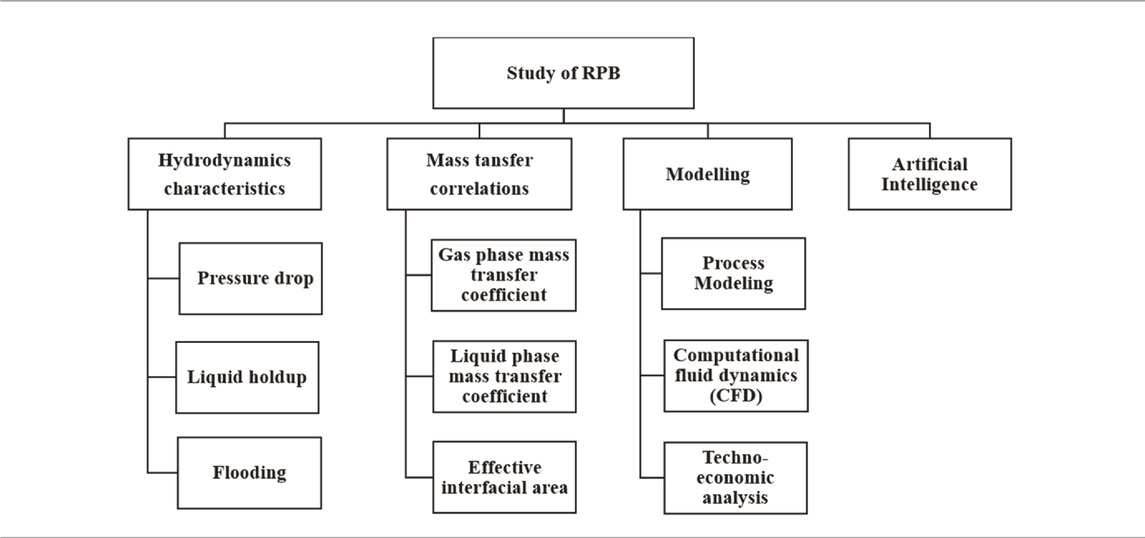
Various factors such as the rotational speed of RPB, CO2 partial pressure, liquid-gas flow rate, the temperature of the liquid phase, absorbent concentration, and CO2 loading solvent affect the working of an RPB. Numerous modeling techniques based on hydrodynamic analysis and the study of mass transfer characteristics using different operational parameters can be used to examine an RPB. A summary of these techniques is presented in Table 1. The analysis of fluid flow behavior in terms of the liquid holdup, flooding, pressure drop, and liquid flow pattern constitutes the hydrodynamic characteristics. The effect of mass transfer properties of the RPB can be investigated by both gas and liquid phase mass transfer coefficients and effective interfacial area. A numerical approach has been applied to study the process modeling of RPB. Different computational fluid dynamics (CFD) approaches, such as Eulerian-Eulerian or Eulerian-Largrangian, have been used to imitate the gas flow state, fluid drop path, and mass transfer coefficients in an RPB (Zhao et al., 2016). Furthermore, studies have analyzed turbulence models such as k-
Additionally, researchers have also developed machine learning or artificial intelligence to simulate absorption in an RPB using an ANN (artificial neural network). The experimental and modeling approaches can be time-consuming at times. As a result, the ANN approach can be involved. An ANN can be used to predict the coefficient of mass transfer, hydrodynamic parameters, and thermodynamic properties of CO2 in the absorption process (W. Li et al., 2016; Z. W; Liu et al., 2018; Zhao et al., 2014). Furthermore, expertise in techno-economic analysis is required to grasp the advantage of the proposed project, taking both benefits and costs into account. (T. L. Chen et al., 2020).
2.1 Hydrodynamics parameters
A thorough understanding of hydrodynamic parameters is vital for improving the performance of an RPB system. As a result of the high rotational conditions, the hydrodynamics of the RPBs are altered in ways that significantly affect the gas-liquid mass transfer coefficient. The effect occurs by changing the RPB’s hydrodynamics, flooding, liquid holdup, pressure drop, and fluid dispersion.
2.1.1 Liquid holdup (
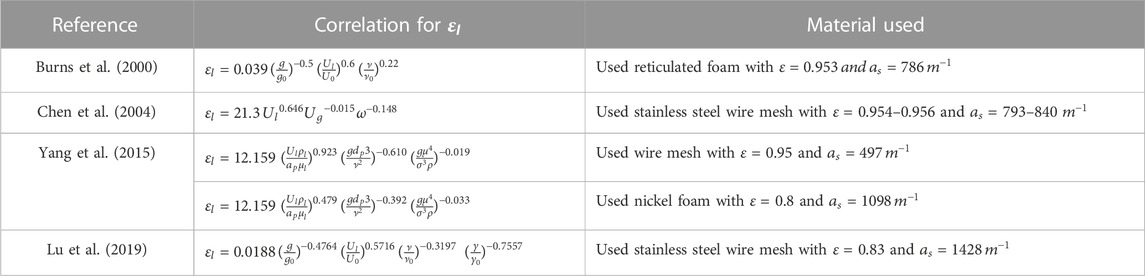
Liquid holdup describes the percentage of liquid volume in the bed after draining. Different correlations have been considered in the literature through modeling depending on the packing type used and flow theory (either film flow or droplet flow). The correlations were then validated with experimental data. These correlations deviate from the existing correlations for conventional columns because of the rotational flow in the RPB.
Basic and Dudukovic (Basic and Dudukovic, n.d.), working under the premise of film flow with complete wetting, presented a correlation for the liquid holdup. It was based on experimental data measured with the electrical resistance measurement method. The research discovered that this expression corresponds with the holdup data as a function of the operational parameters. Furthermore, (Burns et al., 2000) studied the
2.1.2 Pressure-drop (
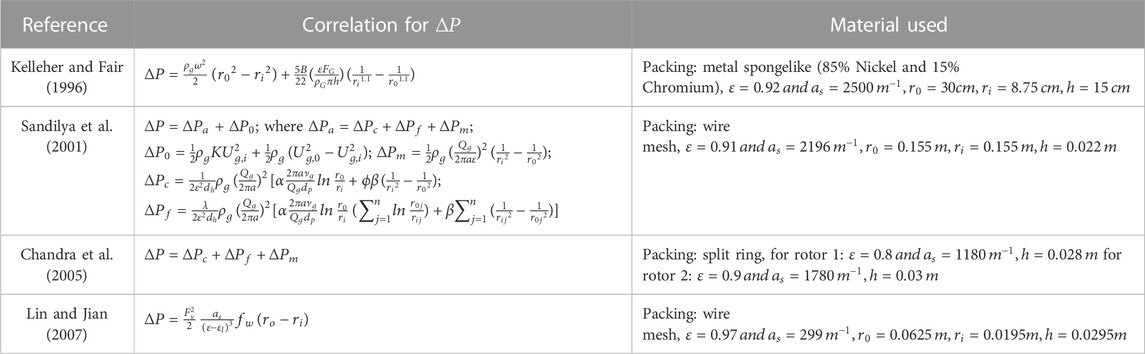
Pressure drop indicates the resistance a fluid encounters due to friction or other forces acting on it, such as gravity, and changes with the type of packing. Studies have been made on the empirical correlations of pressure drop with different radii, porosity, interfacial area, and type of packing. Correlations have been developed using these parameters. Keyvani (Keyvani and Gardner, n.d.) used a momentum integral balance on the rotor and the area between the rotor and container to simulate pressure drop. It was observed that
Here, correlations are derived for a specific RPB with different structures and packing materials. The efficacy of the models or correlations to investigate pressure drops in various RPBs is still to be validated.
2.1.3 Flooding
Flooding occurs when the liquid stops flowing down the column due to the relative vapor flow rates being such that the drag force is higher than or equal to the gravitational force. The flooding phenomenon is different for an RPB compared to conventional columns due to differences in flow area and centrifugal acceleration (Rao et al., 2004). Due to the increased artificial gravity, RPBs may reach larger flooding capacities than conventional columns (Hendry et al., 2020). Two correlations have been used to study flooding: Sherwood’s correlation for the packed column and Lockett’s correlation for RPB.
Sherwood’s approach (Sherwood et al., 1938):
Wallis’s approach (Wallis, 1969) included some more parameters and proposed a relation:
Lockett’s approach (Lockett, 1995) for an RPB has the form:
2.2 Mass transfer correlations
Mass transfer is defined as mass movement from one phase to another. The main reason behind using an RPB instead of the conventional column is its improved mass transfer efficiency. The mass transfer depends on the motion of fluid and hydrodynamic characteristics. Mass transfer of species changes with reaction, time, and space; hence, different mass transfer correlations are used to predict the mass flux of the process. Mathematical modeling of the RPB system requires studying the system of equations based on energy and mass balance, which uses a mass transfer coefficient in the gas-liquid phase and an effective interfacial area. Penetration theory, surface renewal theory, and two film theory are the three theories used to model mass transfer. Based on these theories, different empirical correlations are studied in the literature.
2.2.1 Gas phase mass transfer coefficient
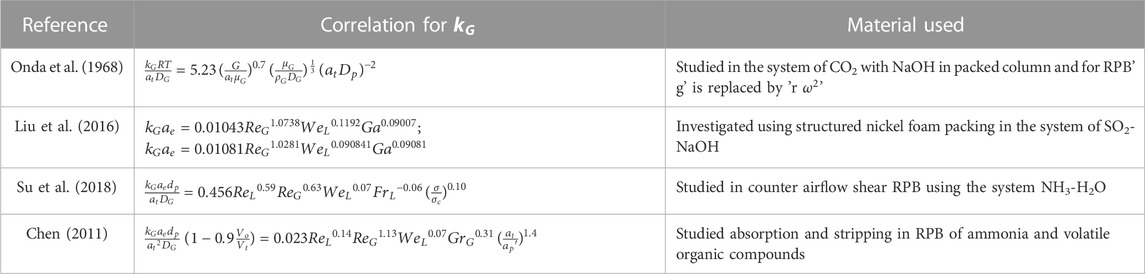
Y. S. Chen, (2011) developed a correlation utilizing the two-film theory. The bulk of the data could be accurately predicted using this correlation. The impacts of the flow rates of gas and liquid, the viscosity of the liquid, and centrifugal acceleration on
2.2.2 Liquid phase mass transfer coefficient
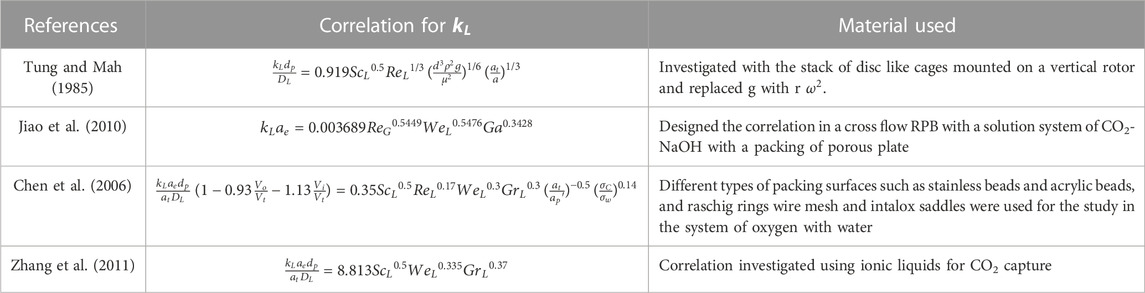
Absorption usually occurs in liquid, hence the
2.2.3 Effective interfacial surface area
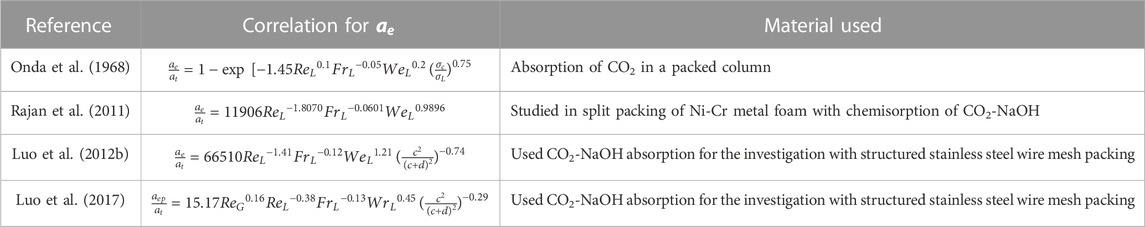
Effective interfacial surface area
The correlation of
2.3 Process modeling review
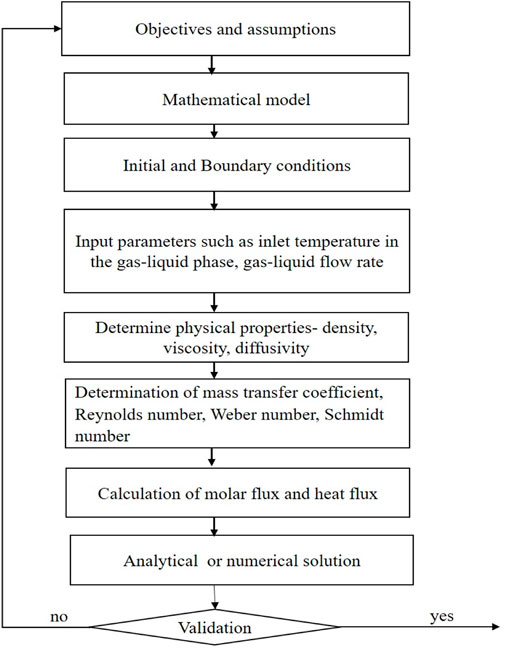
Process modeling is a technique used to predict the behavior of a system through computer techniques and graphical representation. It is further used to understand the influence of parameters on the system. Developing an RPB model involves chemical reaction equations, rate constants, and relations for calculating physical properties. These equations are included in the process modeling algorithm using tools for simulation and analysis and are shown here through a flowchart in Figure 2. For an RPB, a process model will be helpful for design, scaling up, debugging, process monitoring, mechanistic understanding, and optimal design and process evaluation. RPB modeling studied by various researchers is reviewed in this section.
The assumptions made for the model and the calculation of the operational properties are different in different modeling studies. Since the shape of an RPB is circular, the common assumptions made by the researchers are that the flow of fluids is in counter-current and radial directions, shown in Figures 1A, B. To simulate the RPB, distinct theories have been proposed for mass transfer, such as penetration theory, film theory, and surface renewal theory. Sun et al. (2009) established a mathematical system for the absorption of CO2 and NH3 in water for an RPB. The mass equation of CO2 in a droplet, as well as the film zone, was expressed in terms of a partial differential equation with Neumann boundary conditions. Through the model, an overall volumetric mass transfer coefficient
The reliability of these models is significantly dependent on the availability and effectiveness of existing correlations. As a result, developing trustworthy correlations is the foundation for adequately simulating the process using different modeling software. Various correlations with regard to mass transfer are tabulated in Tables 4, 5. The relation shows the internal connection of distinct operational parameters, physical properties of the process, and dimensionless numbers using the different packing materials. Other researchers studied the correlations of
2.4 Review of experimental data with RPB
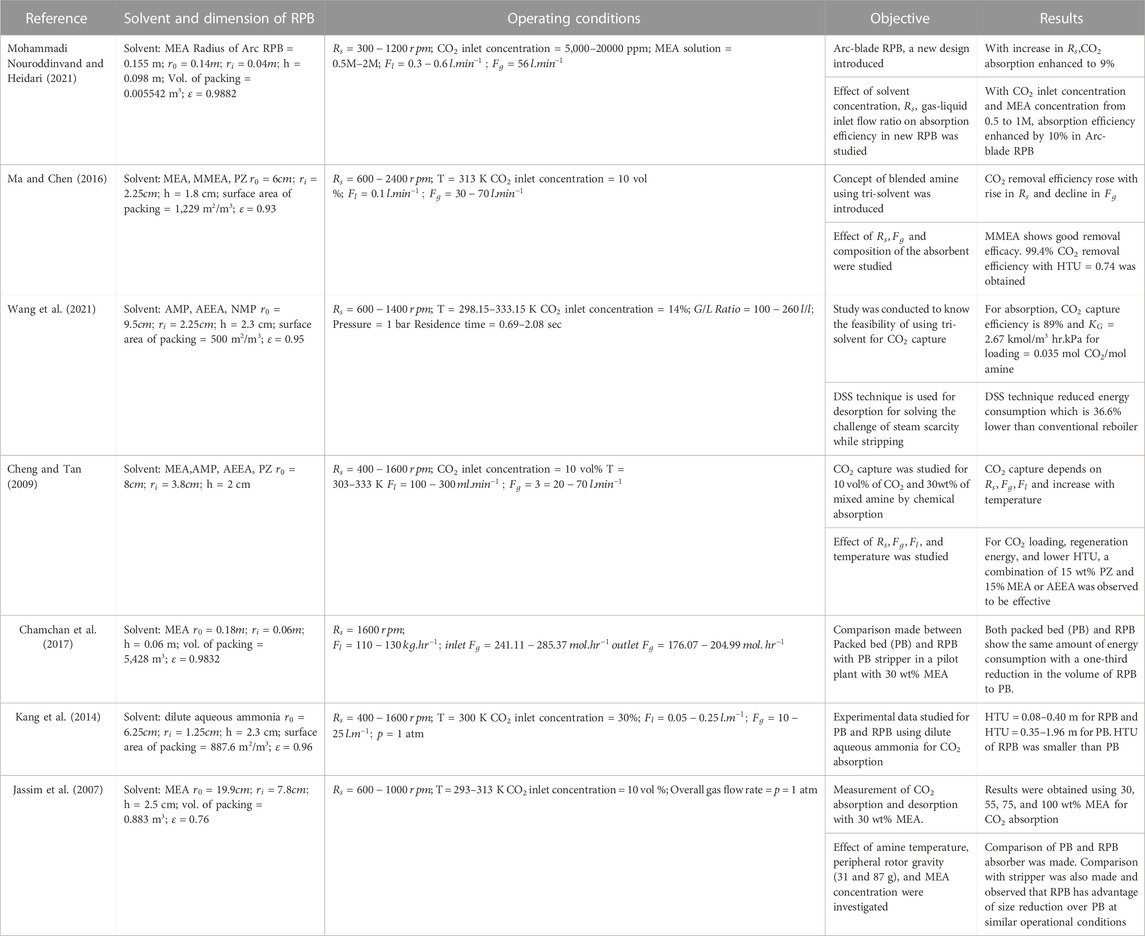
In recent years, for the reduction of CO2 emissions, a PCC process with the RPB technique has been investigated. Many researchers are working to determine CO2 absorption efficiency in an RPB with different packing materials, dimensions of the equipment, and operational parameters. An experiment on the chemical absorption of CO2 was conducted by Cheng and Tan, (2009) in an RPB utilizing a combination of amine solvents (MEA, AEEA, PZ, and AMP). The researchers found that higher temperatures increased absorption efficiency (303–333 K). It was also shown that a higher percentage of PZ in the mixture results in higher capture efficiency. For CO2 absorption, Mohammadi Nouroddinvand and Heidari, (2021) performed experimental results employing an Arc-blade RPB in MEA (0.5–2.0 M) solvent. Wire mesh in the stainless-steel packaging was used. Two rectangular gas inlets, each 75 mm in length and 3 mm wide, were employed instead of circular designs. The obtained efficiency for capturing CO2 was 49%–78%, with
2.5 Techno-economic analysis
Implementing an RPB helps lower operating costs and environmental impact by reducing reactor size and capital expense. There are few studies in the literature that compare the environmental and economic effects of the RPB process to a conventional column process. There are benefits of using an RPB over the conventional column. Joel et al. (2014) compared conventional columns and RPBs and found that reduction in size had a crucial influence on absorption efficiency. Cheng et al. (2013) investigated the regenerator’s regeneration energy and found that an RPB with just a 10th of the volume of a conventional column could achieve the same regeneration efficiency. As a result of the enhanced heat transfer zone in the RPB, less vapor lean MEA needed to be transferred from the reboiler to the RPB. R. Zhang et al. (2017) used three mixed solvents MEA-MDEA-PZ, to study CO2 absorption and desorption. An increase in the MDEA/PZ mole fraction among these blends resulted in a reduction in energy use and an increase in the rate of CO2 absorption. They also looked at the MEA-MDEA-PZ combination, which compared to 5M MEA, used 15.22%–49.92% less energy. M. Wang et al. (2015) noted that an RPB has good mass transport properties and is the most suited-technique for PCC. The performance of an RPB was methodically assessed from the engineering, environmental, economic, and energy perspectives by T. L. Chen et al. (2020). To propose a superior technical option compared to current CCSU facilities, a cost-benefit analysis was carried out utilizing running costs, carbon credit, and reductions in air quality. According to (Otitoju et al., 2023), packed bed absorbers’ and strippers’ size adds to the problems. If the absorbers and strippers can be replaced with RPB equivalents, CO2 collection could be better and cheaper. A rate-based model in the steady state of the RPB absorber was proposed and verified for this study using Aspen Custom Modeller. It was used to perform technical and economic evaluations of operating big RPB absorbers using concentrated MEA (55–75 wt%). Compared to packed bed absorbers, the RPB absorber reduced the volume by a factor of 4–11 at a concentration of 55 wt% MEA, according to the technical evaluations, and had lower capital costs while having the largest volume reduction factors (3%–53% lower).
In conclusion, based on thorough and precise process models, it is necessary to estimate the entire costs (capital and operational expenses) utilized to capture CO2 in intensified and traditional PCC processes. Different modeling approaches have been discussed in detail. The conclusion reached in this context is that the solution of the model for carbon absorption depends on the conservation of mass and energy balance equation with appropriate model assumptions and using appropriate relations for simplifying and solving the system of equations. The CO2 mole fraction is predicted in outlet gas at numerous liquid flow rates, rotational speeds, and temperatures. The impact of different operating factors on the coefficient of mass transfer in RPB can be anticipated with the aid of a model. In addition to describing how mass transfer intensification occurs in an RPB, the gas-liquid mass transfer characteristic mathematical model also provides a theoretical foundation for the creation of RPBs and their actual application. Techno-economic analysis was discussed, which is necessary to optimize the entire expense utilized in CO2 capture with an RPB having advantages over the conventional column. In chemical absorption, further research is needed to reduce solvent degradation, reducing the requirement for solvent make-up and, consequently, decreasing operational costs. New configurations and integration arrangements will contribute to cutting down energy consumption and, ultimately, minimizing expenses. Additives, for example, may be used to decrease surface tension and contact angles and thereby increase absorption rates utilizing CO2 transfer and solvent wetting. The challenges that remain are actively being pursued across the policy, economic, environmental, and technology communities at all levels.
From the viewpoint of reducing emissions of CO2 into the atmosphere, carbon capture utilization and sequestration (CCUS) technology needs to be retrofitted into power plants. Research has been done on the modeling and simulation of CO2 capture for the conventional column, but an RPB is more efficient. More research is required in this domain to make the process of CO2 capture faster with a lower cost. In the future, more modeling attempts are necessary for RPB absorbers in a steady and dynamic state with different solvents. Better operational parameters which influence the mass transfer of fluids are being investigated, which need to be studied to enhance the predictions of mass flux and CO2 absorption efficiency. Furthermore, factors that influence the cost of the process need to be investigated for techno-economic analysis so that the process of CO2 capture also becomes cost-effective. Utilizing CFD and an ANN are also promising approaches for studying hydrodynamic characteristics and mass transfer. The theoretical analysis and modeling of the RPB regenerator is the new scope of research work required. Understanding the complete absorption and desorption process through absorber and desorber together will scale up the CO2 capture process.
3 Conclusion and future scope
RPB process intensification technology for the PCC process fits under sustainable development and industrial decarbonization. An RPB is a better option for CO2 capture due to its advantages compared to the conventional column. However, it still has some disadvantages, such as large pressure drops and other mechanical wear and tear issues. The conventional column requires a considerable height, high capital cost, and more energy efficiency to regenerate the solvent. This equally applies to an RPB as it is a regenerative chemical absorption process. Both processes require energy-efficient solvents for cost-effective CO2 capture. Because the factors that affect the CO2 absorption efficiency depend on hydrodynamic studies, mass transfer correlations, rotating speed, solvent characteristics, and fluid flow rates, this article reviews all these factors. The correlation of hydrodynamic parameters, mass transfer in the gas-liquid phase in RPBs, and process modeling of large-scale RPBs studied in the literature will help to determine the efficiency of CO2 absorption in any amine-based solvent using RPB technology. A reasonable interpretation of the whole process can thus be made. The centrifugal surroundings have a significant influence on mass transport by modifying the hydrodynamics of an RPB. As a result, a thorough grasp of this is required to improve an RPB’s mass transfer performance.
Different hydrodynamic characteristics were discussed. The distinct correlations of liquid holdup and pressure drop in RPBs were summarised and showed that the parameters that affect pressure drop are rotational speed and centrifugal acceleration. Furthermore, studies were made to determine the influence of flow rates on pressure drop. The parameters that affect liquid holdup depend on velocity, rotor speed, and the packing structure. Hence, correlations using these parameters were discussed. The understanding of mass transfer is essential as it has a significant effect on the absorption of CO2. Experimental studies with MEA and other amines/blended amines were explored. As pilot plant and scale-up studies are essential, this review provides a direction for a future CO2 capture study in a pilot process with an energy-efficient solvent at the CO2 research center of our university.
Author contributions
CS: Analysis and interpretation; methodology; writing–original draft; review and editing. SD: Conceptualization; resources; project administration, supervision writing—review and editing. PM: Supervision; validation; writing review and editing.
Acknowledgments
The authors are thankful for financial support from the Department of Biotechnology (DBT), Ministry of Science and Technology, Govt. of India; through the project “Integrated Design and Demonstration of Intensified CO2 Capture with cost-effective advanced Process. (INDIA-CO2),” No. T/PR31120/PBD/26/755/2019.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afkhamipour, M., and Mofarahi, M. (2013). Comparison of rate-based and equilibrium-stage models of a packed column for post-combustion CO2 capture using 2-amino-2-methyl-1-propanol (AMP) solution. Int. J. Greenh. Gas Control 15, 186–199. doi:10.1016/j.ijggc.2013.02.022
Akanksha, , Pant, K. K., and Srivastava, V. K. (2007). Carbon dioxide absorption into monoethanolamine in a continuous film contactor. Chem. Eng. J. 133 (1–3), 229–237. doi:10.1016/j.cej.2007.02.001
Ayash, A. A., and Mahmood, M. A. (2022). Conventional and non-conventional gas-liquid contacting methods: A critical review and a quantitative evaluation. AIP Conf. Proc. 2660. doi:10.1063/5.0107723
Basic, A., and Dudukovic, M. P. (n.d.). Liquid holdup in rotating packed beds: Examination of the film flow assumption. AIChE J. 41 (2), 301–316. doi:10.1002/aic.690410212
Billet, R., and Schultes, M. (1999). Prediction of mass transfer columns with dumped and arranged packings: Updated summary of the calculation method of Billet and Schultes. Chem. Eng. Res. Des. 77 (6), 498–504. doi:10.1205/026387699526520
Borhani, T. N., Oko, E., and Wang, M. (2018). Process modelling and analysis of intensified CO2 capture using monoethanolamine (MEA) in rotating packed bed absorber. J. Clean. Prod. 204, 1124–1142. doi:10.1016/j.jclepro.2018.09.089
Burns, J. R., Jamil, J. N., and Ramshaw, C. (2000). Process intensification: Operating characteristics of rotating packed beds - determination of liquid hold-up for a high-voidage structured packing. Chem. Eng. Sci. 55 (13), 2401–2415. doi:10.1016/S0009-2509(99)00520-5
Chamchan, N., Chang, J. Y., Hsu, H. C., Kang, J. L., Wong, D. S. H., Jang, S. S., et al. (2017). Comparison of rotating packed bed and packed bed absorber in pilot plant and model simulation for CO2 capture. J. Taiwan Inst. Chem. Eng. 73, 20–26. doi:10.1016/j.jtice.2016.08.046
Chandra, A., Goswami, P. S., and Rao, D. P. (2005). Characteristics of flow in a rotating packed bed (HIGEE) with split packing. Industrial Eng. Chem. Res. 44 (11), 4051–4060. doi:10.1021/ie048815u
Chen, T. L., Pei, S. L., Pan, S. Y., Yu, C. Y., Chang, C. L., and Chiang, P. C. (2020). An engineering-environmental-economic-energy assessment for integrated air pollutants reduction, CO2 capture and utilization exemplified by the high-gravity process. J. Environ. Manag. 255, 109870. doi:10.1016/j.jenvman.2019.109870
Chen, Y. H., Chang, C. Y., Su, W. L., Chen, C. C., Chiu, C. Y., Yu, Y. H., et al. (2004). Modeling ozone contacting process in a rotating packed bed. Industrial Eng. Chem. Res. 43 (1), 228–236. doi:10.1021/ie030545c
Chen, Y. S. (2011). Correlations of mass transfer coefficients in a rotating packed bed. Industrial Eng. Chem. Res. 50 (3), 1778–1785. doi:10.1021/ie101251z
Chen, Y. S., Lin, C. C., and Liu, H. S. (2005). Mass transfer in a rotating packed bed with various radii of the bed. Industrial Eng. Chem. Res. 44 (20), 7868–7875. doi:10.1021/ie048962s
Chen, Y. S., Lin, F. Y., Lin, C. C., Tai, C. Y. der, and Liu, H. S. (2006). Packing characteristics for mass transfer in a rotating packed bed. Industrial Eng. Chem. Res. 45 (20), 6846–6853. doi:10.1021/ie060399l
Cheng, H. H., Lai, C. C., and Tan, C. S. (2013). Thermal regeneration of alkanolamine solutions in a rotating packed bed. Int. J. Greenh. Gas Control 16, 206–216. doi:10.1016/j.ijggc.2013.03.022
Cheng, H. H., and Tan, C. S. (2009). Carbon dioxide capture by blended alkanolamines in rotating packed bed. Energy Procedia 1 (1), 925–932. doi:10.1016/j.egypro.2009.01.123
Chu, G. W., Sang, L., Du, X. K., Luo, Y., Zou, H. K., and Chen, J. F. (2015). Studies of CO2 absorption and effective interfacial area in a two-stage rotating packed bed with nickel foam packing. Chem. Eng. Process. Process Intensif. 90, 34–40. doi:10.1016/j.cep.2015.02.007
Cortes Garcia, G. E., van der Schaaf, J., and Kiss, A. A. (2017). A review on process intensification in HiGee distillation. J. Chem. Technol. Biotechnol. 92 (6), 1136–1156. doi:10.1002/jctb.5206
Dash, S. K., Parikh, R., and Kaul, D. (2022). Development of efficient absorbent for CO2 capture process based on (AMP + 1MPZ). Mater. Today Proc. 62 (P13), 7072–7076. doi:10.1016/j.matpr.2022.01.148
Dash, S. K., Samanta, A. N., and Bandyopadhyay, S. S. (2012). Experimental and theoretical investigation of solubility of carbon dioxide in concentrated aqueous solution of 2-amino-2-methyl-1-propanol and piperazine. J. Chem. Thermodyn. 51, 120–125. doi:10.1016/j.jct.2012.02.012
Dey, A., Dash, S. K., and Mandal, B. (2018). Equilibrium CO2 solubility and thermophysical properties of aqueous blends of 1-(2-aminoethyl) piperazine and N-methyldiethanolamine. Fluid Phase Equilibria 463, 91–105. doi:10.1016/j.fluid.2018.01.030
Dey, A., Mandal, B., and Dash, S. K. (2020). Analysis of equilibrium CO2 solubility in aqueous APDA and its potential blends with AMP/MDEA for postcombustion CO2 capture. Int. J. Energy Res. 44 (15), 12395–12415. doi:10.1002/er.5404
Dhaneesh, K. P., and Ranganathan, P. (2022). A comprehensive review on the hydrodynamics, mass transfer and chemical absorption of CO2 and modelling aspects of rotating packed bed. Sep. Purif. Technol. 295, 121248. doi:10.1016/j.seppur.2022.121248
Esmaeili, A., Tamuzi, A., Borhani, T. N., Xiang, Y., and Shao, L. (2022). Modeling of carbon dioxide absorption by solution of piperazine and methyldiethanolamine in a rotating packed bed. Chem. Eng. Sci. 248, 117118. doi:10.1016/j.ces.2021.117118
Gao, X. Y., Liu, L., Hu, M. L., Xiang, Y., Chu, G. W., Zou, H. K., et al. (2016). Numerical simulation for mass transfer characteristics of CO2 capture in a rotating packed bed. Chem. Eng. Process. Process Intensif. 109, 68–79. doi:10.1016/j.cep.2016.08.015
Guo, J., Jiao, W., Qi, G., Yuan, Z., and Liu, Y. (2019). Applications of high-gravity technologies in gas purifications: A review. Chin. J. Chem. Eng. 27 (6), 1361–1373. doi:10.1016/j.cjche.2019.01.011
Harun, N., Nittaya, T., Douglas, P. L., Croiset, E., and Ricardez-Sandoval, L. A. (2012). Dynamic simulation of MEA absorption process for CO2 capture from power plants. Int. J. Greenh. Gas Control 10, 295–309. doi:10.1016/j.ijggc.2012.06.017
Hendry, J. R., Lee, J. G. M., and Attidekou, P. S. (2020). Pressure drop and flooding in rotating packed beds. Chem. Eng. Process. - Process Intensif. 151, 107908. doi:10.1016/j.cep.2020.107908
Im, D., Jung, H., and Lee, J. H. (2020). Modeling, simulation and optimization of the rotating packed bed (RPB) absorber and stripper for MEA-based carbon capture. Comput. Chem. Eng. 143, 107102. doi:10.1016/j.compchemeng.2020.107102
IPCC (2022). Climate Change 2022: Impacts, adaptation, and vulnerability. Contribution of working group II to the sixth assessment report of the IPCC. Cambridge, United Kingdom and New York, NY: Cambridge University Press, 3056. doi:10.1017/9781009325844
Jassim, M. S. (2002). Process intensification: Absorption and desorption of carbon dioxide from monoethanolamine solutions using higee technology. Newcastle, England: Newcastle University.
Jassim, M. S., Rochelle, G., Eimer, D., and Ramshaw, C. (2007). Carbon dioxide absorption and desorption in aqueous monoethanolamine solutions in a rotating packed bed. Industrial Eng. Chem. Res. 46 (9), 2823–2833. doi:10.1021/ie051104r
Jiao, W. Z., Liu, Y. Z., and Qi, G. S. (2010). Gas pressure drop and mass transfer characteristics in a cross-flow rotating packed bed with porous plate packing. Industrial Eng. Chem. Res. 49 (8), 3732–3740. doi:10.1021/ie9009777
Jin, H., Li, J., Liu, P., and Li, Z. (2019). Rate-based modelling and validation of an absorber and stripper in an amine-based post-combustion CO2 capture process. Chem. Eng. Trans. 76, 811–816. doi:10.3303/CET1976136
Joel, A. S., Wang, M., and Ramshaw, C. (2015). Modelling and simulation of intensified absorber for post-combustion CO2 capture using different mass transfer correlations. Appl. Therm. Eng. 74, 47–53. doi:10.1016/j.applthermaleng.2014.02.064
Joel, A. S., Wang, M., Ramshaw, C., and Oko, E. (2014). Process analysis of intensified absorber for post-combustion CO2 capture through modelling and simulation. Int. J. Greenh. Gas Control 21, 91–100. doi:10.1016/j.ijggc.2013.12.005
Kang, J. L., Sun, K., Wong, D. S. H., Jang, S. S., and Tan, C. S. (2014). Modeling studies on absorption of CO2 by monoethanolamine in rotating packed bed. Int. J. Greenh. Gas Control 25, 141–150. doi:10.1016/j.ijggc.2014.04.011
Kelleher, T., and Fair, J. R. (1996). Distillation studies in a high-gravity contactor. Ind. Eng. Chem. Res. 35 (12), 4646–4655. doi:10.1021/ie950662a
Keyvani, M., and Gardner, N. C. (1989). Operating characteristics of rotating beds. Chem. Eng. Prog. 85 (9), 48–52.
Koronaki, I. P., Prentza, L., and Papaefthimiou, V. (2015). Modeling of CO2 capture via chemical absorption processes - an extensive literature review. Renew. Sustain. Energy Rev. 50, 547–566. doi:10.1016/j.rser.2015.04.124
Kvamsdal, H. M., Jakobsen, J. P., and Hoff, K. A. (2009). Dynamic modeling and simulation of a CO2 absorber column for post-combustion CO2 capture. Chem. Eng. Process. Process Intensif. 48 (1), 135–144. doi:10.1016/j.cep.2008.03.002
Li, H., Li, L., Nguyen, T., Rochelle, G. T., and Chen, J. (2013). Characterization of piperazine/2-aminomethylpropanol for carbon dioxide capture. Energy Procedia 37, 340–352. doi:10.1016/j.egypro.2013.05.120
Li, W., Wei, S., Jiao, W., Qi, G., and Liu, Y. (2016). Modelling of adsorption in rotating packed bed using artificial neural networks (ANN). Chem. Eng. Res. Des. 114, 89–95. doi:10.1016/j.cherd.2016.08.013
Li, Y., Lu, Y., Liu, X. J., Wang, G., Nie, Y., and Ji, J. (2017). Mass-transfer characteristics in a rotating zigzag bed as a Higee device. Sep. Purif. Technol. 186, 156–165. doi:10.1016/j.seppur.2017.05.049
Lin, C. C., and Jian, G. S. (2007). Characteristics of a rotating packed bed equipped with blade packings. Sep. Purif. Technol. 54 (1), 51–60. doi:10.1016/j.seppur.2006.08.006
Lin, C. C., and Kuo, Y. W. (2016). Mass transfer performance of rotating packed beds with blade packings in absorption of CO2 into MEA solution. Int. J. Heat Mass Transf. 97, 712–718. doi:10.1016/j.ijheatmasstransfer.2016.02.033
Liu, Y., Zhang, F., Gu, D., Qi, G., Jiao, W., and Chen, X. (2016). Gas-phase mass transfer characteristics in a counter airflow shear rotating packed bed. Can. J. Chem. Eng. 94 (4), 771–778. doi:10.1002/cjce.22434
Liu, Z. W., Liang, F. N., and Liu, Y. Z. (2018). Artificial neural network modeling of biosorption process using agricultural wastes in a rotating packed bed. Appl. Therm. Eng. 140, 95–101. doi:10.1016/j.applthermaleng.2018.05.029
Lockett, M. J. (1995). Flooding of rotating structured packing and its application to conventional packed-columns. Chem. Eng. Res. Des. 73, 379–984.
Lu, X., Xie, P., Ingham, D. B., Ma, L., and Pourkashanian, M. (2019). Modelling of CO2 absorption in a rotating packed bed using an Eulerian porous media approach. Chem. Eng. Sci. 199, 302–318. doi:10.1016/j.ces.2019.01.029
Luo, Y., Chu, G. W., Zou, H. K., Wang, F., Xiang, Y., Shao, L., et al. (2012a). Mass transfer studies in a rotating packed bed with novel rotors: Chemisorption of CO 2. Industrial Eng. Chem. Res. 51 (26), 9164–9172. doi:10.1021/ie300466f
Luo, Y., Chu, G. W., Zou, H. K., Zhao, Z. Q., Dudukovic, M. P., and Chen, J. F. (2012b). Gas-liquid effective interfacial area in a rotating packed bed. Industrial Eng. Chem. Res. 51 (50), 16320–16325. doi:10.1021/ie302531j
Luo, Y., Luo, J. Z., Chu, G. W., Zhao, Z. Q., Arowo, M., and Chen, J. F. (2017). Investigation of effective interfacial area in a rotating packed bed with structured stainless steel wire mesh packing. Chem. Eng. Sci. 170, 347–354. doi:10.1016/j.ces.2016.10.023
Ma, H. J., and Chen, Y. S. (2016). Evaluation of effectiveness of highly concentrated alkanolamine solutions for capturing CO2 in a rotating packed bed. Int. J. Greenh. Gas Control 55, 55–59. doi:10.1016/j.ijggc.2016.11.009
Mallinson, R. H., and Ramshaw, C. (1981). Mass transfer process. Patent No. 4,283,255. United States: United States Patent.
Marx-Schubach, T., and Schmitz, G. (2019). Modeling and simulation of the start-up process of coal fired power plants with post-combustion CO2 capture. Int. J. Greenh. Gas Control 87, 44–57. doi:10.1016/j.ijggc.2019.05.003
Mohammadi Nouroddinvand, V., and Heidari, A. (2021). Experimental study of CO2 absorption with MEA solution in a novel Arc-RPB. Chem. Eng. Process. - Process Intensif. 165, 108450. doi:10.1016/j.cep.2021.108450
Neumann, K., Gladyszewski, K., Groß, K., Qammar, H., Wenzel, D., Górak, A., et al. (2018). A guide on the industrial application of rotating packed beds. Chem. Eng. Res. Des. 134, 443–462. doi:10.1016/j.cherd.2018.04.024
Onda, K., Sada, E., and Takeuchi, H. (1968). Gas absorption with chemical reaction in packed columns. Sammak Trans. Inst. Chem. Engrs 36 (9), 62–66. doi:10.1252/jcej.1.62
Otitoju, O., Oko, E., and Wang, M. (2023). Modelling, scale-up and techno-economic assessment of rotating packed bed absorber for CO2 capture from a 250 MWe combined cycle gas turbine power plant. Appl. Energy 335, 120747. doi:10.1016/j.apenergy.2023.120747
Pan, S. Y., Wang, P., Chen, Q., Jiang, W., Chu, Y. H., and Chiang, P. C. (2017). Development of high-gravity technology for removing particulate and gaseous pollutant emissions: Principles and applications. J. Clean. Prod. 149, 540–556. doi:10.1016/j.jclepro.2017.02.108
Pandya, J. D. (1983). Adiabatic gas absorption and stripping with chemical reaction in packed towers. Chem. Eng. Commun. 19 (4–6), 343–361. doi:10.1080/00986448308956351
Podbielniak, W. J. (1935). Centrifugal counter current contact apparatus. United States: US patent 2,004,011.
Qian, Z., Xu, L., Cao, H., and Guo, K. (2009). Modeling study on absorption of CO2 by aqueous solutions of n-methyldiethanolamine in rotating packed bed. Industrial Eng. Chem. Res. 48 (20), 9261–9267. doi:10.1021/ie900894a
Rajan, S., Kumar, M., Ansari, M. J., Rao, D. P., and Kaistha, N. (2011). Limiting gas liquid flows and mass transfer in a novel rotating packed bed (HiGee). Industrial Eng. Chem. Res. 50 (2), 986–997. doi:10.1021/ie100899r
Rao, D. P., Bhowal, A., and Goswami, P. S. (2004). Process intensification in rotating packed beds (HIGEE): An appraisal. Industrial Eng. Chem. Res. 43 (4), 1150–1162. doi:10.1021/ie030630k
Rao, D. P. (2022). Commentary: Evolution of high gravity (HiGee) technology. Industrial Eng. Chem. Res. 61 (2), 997–1003. doi:10.1021/acs.iecr.1c04587
Reay, D. (2008). The role of process intensification in cutting greenhouse gas emissions. Appl. Therm. Eng. 28 (16), 2011–2019. doi:10.1016/j.applthermaleng.2008.01.004
Sandilya, P., Rao, D. P., Sharma, A., and Biswas, G. (2001). Gas-phase mass transfer in a centrifugal contactor. Industrial Eng. Chem. Res. 40 (1), 384–392. doi:10.1021/ie0000818
Shahid, M. Z., Maulud, A. S., Bustam, M. A., Suleman, H., Abdul Halim, H. N., and Shariff, A. M. (2021). Packed column modelling and experimental evaluation for CO2 absorption using MDEA solution at high pressure and high CO2 concentrations. J. Nat. Gas Sci. Eng. 88, 103829. doi:10.1016/j.jngse.2021.103829
Sherwood, T. K., Shipley, A. N. D, G. H., and Holloway, F. A. L. (1938). Flooding velocities in packed columns. Ind. Eng. Chem. 30 (7), 765–769. doi:10.1021/ie50343a008
Su, M. J., Luo, Y., Chu, G. W., Liu, W., Zheng, X. H., and Chen, J. F. (2018). Gas-side mass transfer in a rotating packed bed with structured nickel foam packing. Industrial Eng. Chem. Res. 57 (13), 4743–4747. doi:10.1021/acs.iecr.8b00269
Sun, B. C., Wang, X. M., Chen, J. M., Chu, G. W., Chen, J. F., and Shao, L. (2009). Simultaneous absorption of CO2 and NH3 into water in a rotating packed bed. Industrial Eng. Chem. Res. 48 (24), 11175–11180. doi:10.1021/ie9001316
Tsai, C. Y., and Chen, Y. S. (2015). Effective interfacial area and liquid-side mass transfer coefficients in a rotating bed equipped with baffles. Sep. Purif. Technol. 144, 139–145. doi:10.1016/j.seppur.2015.02.008
Tung, H. H., and Mah, R. S. H. (1985). Modeling liquid mass transfer in higee separation process. Chem. Eng. Commun. 39 (1–6), 147–153. doi:10.1080/00986448508911667
Wang, M., Joel, A. S., Ramshaw, C., Eimer, D., and Musa, N. M. (2015). Process intensification for post-combustion CO2 capture with chemical absorption: A critical review. Appl. Energy 158, 275–291. doi:10.1016/j.apenergy.2015.08.083
Wang, M., Lawal, A., Stephenson, P., Sidders, J., and Ramshaw, C. (2011). Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 89 (9), 1609–1624. doi:10.1016/j.cherd.2010.11.005
Wang, Y., Dong, Y., Zhang, L., Chu, G., Zou, H., Sun, B., et al. (2021). Carbon dioxide capture by non-aqueous blend in rotating packed bed reactor: Absorption and desorption investigation. Sep. Purif. Technol. 269, 118714. doi:10.1016/j.seppur.2021.118714
Wu, X., Wang, M., Liao, P., Shen, J., and Li, Y. (2020). Solvent-based post-combustion CO2 capture for power plants: A critical review and perspective on dynamic modelling, system identification, process control and flexible operation. Appl. Energy 257, 113941. doi:10.1016/j.apenergy.2019.113941
Xie, P., Lu, X., Yang, X., Ingham, D., Ma, L., and Pourkashanian, M. (2017). Characteristics of liquid flow in a rotating packed bed for CO2 capture: A CFD analysis. Chem. Eng. Sci. 172, 216–229. doi:10.1016/j.ces.2017.06.040
Yang, W., Wang, Y., Chen, J., and Fei, W. (2010). Computational fluid dynamic simulation of fluid flow in a rotating packed bed. Chem. Eng. J. 156 (3), 582–587. doi:10.1016/j.cej.2009.04.013
Yang, Y., Xiang, Y., Chu, G., Zou, H., Luo, Y., Arowo, M., et al. (2015). A noninvasive X-ray technique for determination of liquid holdup in a rotating packed bed. Chem. Eng. Sci. 138, 244–255. doi:10.1016/j.ces.2015.07.044
Yi, F., Zou, H. K., Chu, G. W., Shao, L., and Chen, J. F. (2009). Modeling and experimental studies on absorption of CO2 by Benfield solution in rotating packed bed. Chem. Eng. J. 145 (3), 377–384. doi:10.1016/j.cej.2008.08.004
Zhan, J., Wang, B., Zhang, L., Sun, B. C., Fu, J., Chu, G. W., et al. (2020). Simultaneous absorption of H2S and CO2 into the MDEA + PZ aqueous solution in a rotating packed bed. Industrial Eng. Chem. Res. 59 (17), 8295–8303. doi:10.1021/acs.iecr.9b06437
Zhang, L. L., Wang, J. X., Xiang, Y., Zeng, X. F., and Chen, J. F. (2011). Absorption of carbon dioxide with ionic liquid in a rotating packed bed contactor: Mass transfer study. Industrial Eng. Chem. Res. 50 (11), 6957–6964. doi:10.1021/ie1025979
Zhang, R., Zhang, X., Yang, Q., Yu, H., Liang, Z., and Luo, X. (2017). Analysis of the reduction of energy cost by using MEA-MDEA-PZ solvent for post-combustion carbon dioxide capture (PCC). Appl. Energy 205, 1002–1011. doi:10.1016/j.apenergy.2017.08.130
Zhao, B., Su, Y., and Tao, W. (2014). Mass transfer performance of CO2 capture in rotating packed bed: Dimensionless modeling and intelligent prediction. Appl. Energy 136, 132–142. doi:10.1016/j.apenergy.2014.08.108
Zhao, B., Tao, W., Zhong, M., Su, Y., and Cui, G. (2016). Process, performance and modeling of CO2 capture by chemical absorption using high gravity: A review. Renew. Sustain. Energy Rev. 65, 44–56. doi:10.1016/j.rser.2016.06.059
Nomenclature
AF-1 air flow rate (kmol/s)
F-1 liquid flow rate (kmol/s)
K coefficient of contraction or expansion
P-1 pressure (kPa)
P-2 gas Pressure (kPa)
T-1 liquid inlet temperature (K)
T-2 gas inlet temperature (K)
T-3 gas cooler temperature (K)
T-4 liquid outlet temperature (K)
Dimensionless symbols
Greek symbols
Keywords: carbon capture, chemical absorption, process intensification, rotating packed bed, process modeling
Citation: Shukla C, Mishra P and Dash SK (2023) A review of process intensified CO2 capture in RPB for sustainability and contribution to industrial net zero. Front. Energy Res. 11:1135188. doi: 10.3389/fenrg.2023.1135188
Received: 31 December 2022; Accepted: 17 March 2023;
Published: 13 April 2023.
Edited by:
Hailong Li, Central South University, ChinaReviewed by:
Shufeng Shen, Hebei University of Science and Technology, ChinaTse-Lun Chen, ETH Zürich, Switzerland
Copyright © 2023 Shukla, Mishra and Dash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sukanta Kumar Dash, sk.dash@sot.pdpu.ac.in
 Chetna Shukla
Chetna Shukla Poonam Mishra1 and
Poonam Mishra1 and  Sukanta Kumar Dash
Sukanta Kumar Dash